Enhancing the Best-In-Class Intrasaccular Technology
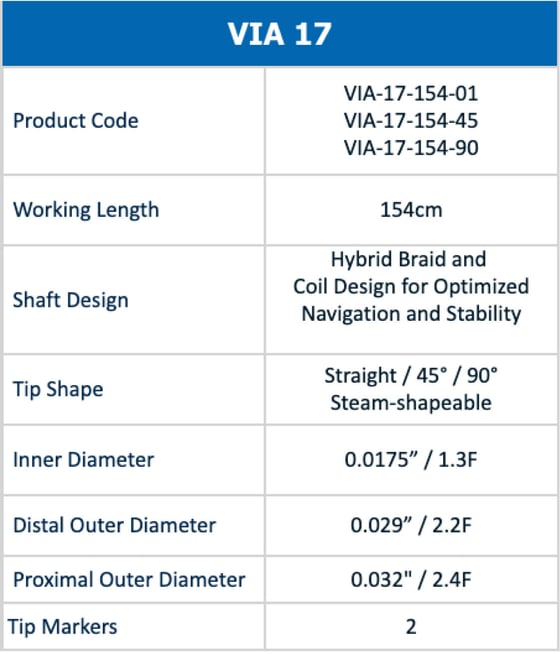

_Page_1.jpg?width=1920&height=1440&name=WEB%20Sizing%20Chart%20-%20US%20(3)_Page_1.jpg)
_Page_2.jpg?width=1920&height=1440&name=WEB%20Sizing%20Chart%20-%20US%20(3)_Page_2.jpg)
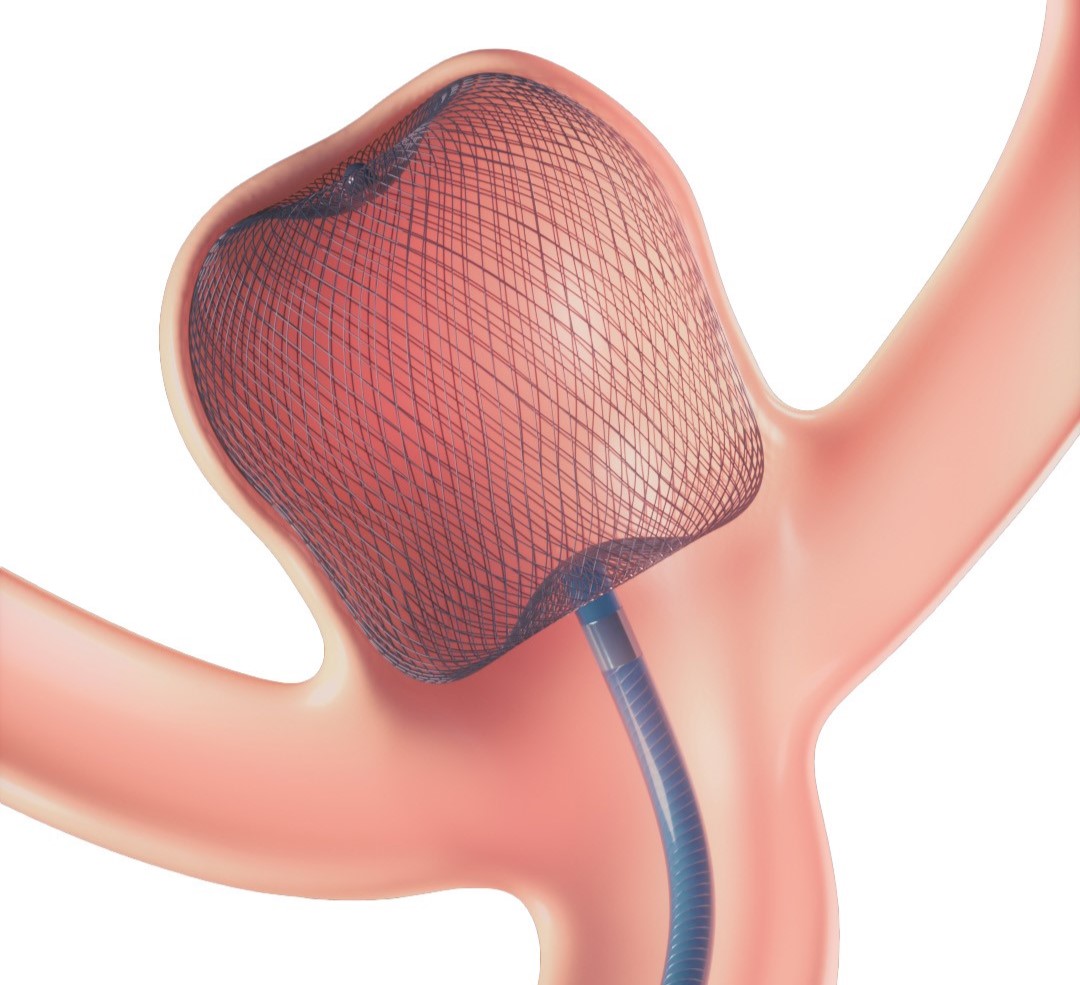
The WEB Device is designed to seal the neck of the aneurysm while also protecting the dome.
The Most Studied Intrasaccular Device
Seven Good Clinical Practice (GCP) Studies and hundreds of publications on its safetyand effectiveness treating a variety of different aneurysms
_Page_5_edited.jpg?height=2000&name=WEB%20Brochure%20-%20US%20(003)_Page_5_edited.jpg)
Established Long Term Clinical Data
2-year follow up confirms high level of safety and occlusion stability, with low procedure-related morbidity and mortality.

Case Examples
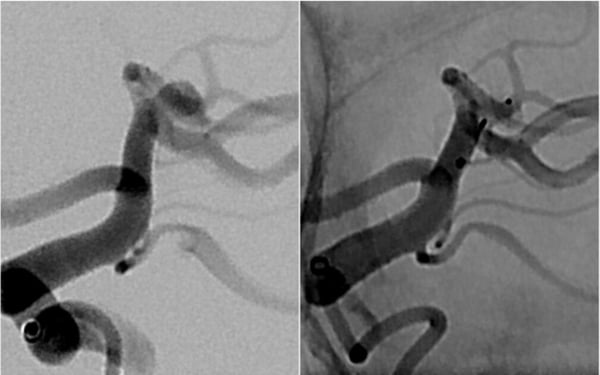
Shallower sizes expand treatment options for patients. WEB 17 SL5x2 implanted in a shallow MCA bifurcation aneurysm.
(Courtesy of Josser Delgado, Yasha Kayan, Alex Copelan)
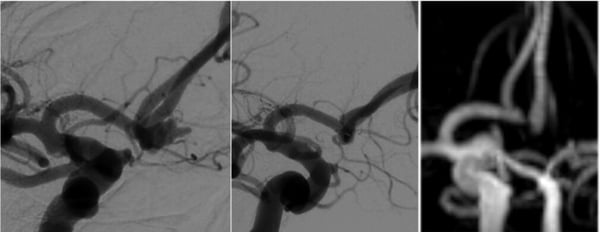
Enhanced navigability of VIA 17 microcatheter and WEB 17 system improves ease of use in ruptured aneurysm cases.
WEB 17 4.5x3 SL implanted in ruptured Acomm aneurysm and 3-month follow-up.
(Courtesy of Josser Delgado, Yasha Kayan, Alex Copelan)
- 1. Van Rooij SBT, Peluso JP, Sluzewski M, Kortman HG, van Rooij WJ. The New Low-Profile WEB 17 System for Treatment of Intracranial Aneurysms: FirstClinical Experiences. AJNR Am J Neuroradiol 2018.
2. Maurer C, Konig I, Berlis A, Weber W,Fischer S. Two-Center Experience in the Endovascular Treatment of Intracranial Aneurysms Using the Woven EndoBridge 17 Device Including Midterm Follow-Up Results: A Retrospective Analysis. AJNR Am J Neuroradiol 2019.
3. Goertz L, Liebig T, Siebert E, et al. Low-Profile Intra-Aneurysmal Flow Disruptor WEB 17 versus WEB Predecessor Systems for Treatment of Small Intracranial Aneurysms: Comparative Analysis of Procedural Safety and Feasibility. AJNR Am J Neuroradiol 2019;40:1766-72
4. Konig I, Maurer C, Berlis A, Maus V, Weber W, Fischer S. Treatment of Ruptured and Unruptured Intracranial Aneurysms with WEB 17 Versus WEB 21 Systems : Comparison of Indications and Early Angiographic Outcomes. Clin Neuroradiol 2020.
Indications, Safety & Warnings
For the full Indications For Use, MRI Safety Information, and Warnings & Precautions, please click HERE.
The WEB Aneurysm Embolization System is indicated for use at the middle cerebral artery (MCA) bifurcation, internal carotid artery (ICA) terminus, anterior communicating artery (AComm) complex, or basilar artery apex for the endovascular treatment of adult patients with saccular, wide neck bifurcation intracranial aneurysms with dome diameter from 3 mm to 10 mm and either neck size 4 mm or greater or the dome-to-neck ratio is greater than 1 and less than 2.
POTENTIAL COMPLICATIONS:
Potential complications include but are not limited to the following: hematoma at the site of entry, aneurysm rupture, emboli, vessel perforation, parent artery occlusion, hemorrhage, ischemia, vasospasm, clotformation, device migration or misplacement, premature or difficult device detachment, non-detachment, incomplete aneurysm filling, revascularization,post-embolization syndrome, and neurological deficits including stroke and death. For complete indications, potential complications, warnings, precautions, and instructions, see instructions for use (IFU provided with thedevice).
VIA 21, 27, 33 - The VIA Microcatheter is intended for the introduction of non-liquid interventional devices (such as aneurysmembolization devices (e.g. WEB device / stents / flow diverters) and infusion of diagnostic (such as contrast media) or non-liquid therapeutic agents into the neuro, peripheral, and coronary vasculature.
VIA 17, 17 Preshaped 45°, 17 Preshaped 90° - The VIA Microcatheter is intended for the introduction of non-liquid interventional devices (such asaneurysm embolization devices (e.g WEB device / coils / stents) and infusion of diagnostic (such as contrast media) or non-liquid therapeutic agents into the neuro, peripheral, and coronary vasculature.
The VIA Microcatheter is contraindicated for use with liquid embolic materials, such as n-butyl 2-cyanoacrylate or ethylene vinyl alcohol & DMSO (dimethyl sulfoxide).
The device should only be used by physicians who have undergone training in all aspects of the WEB Aneurysm Embolization System procedure as prescribed by the manufacturer.
RX Only: Federal law restricts this device to sale by or on the order of a physician.
WEB™ and VIA™ are registered trademarks of MicroVention, Inc. in the United States.
MICROVENTION is a registered trademark of MicroVention, Inc. in the United States and other jurisdictions.
©2023 MicroVention, Inc. MM1140 Rev. A US 12/23
Terumo Neuro Worldwide Innovation Center
35 Enterprise
Aliso Viejo, CA 92656 USA
Phone: 1 (714) 247-8000
Customer Service: 1 (800) 990-8368
microvention.com
